‘Emerade’ adrenaline auto-injector recall
All Emerade adrenaline auto-injector pens are being recalled due to issues that could inhibit their ability to function properly and therefore treat anaphylaxis.
But what is an Emerade? Are they different to EpiPen? What does this all mean?
First, let’s cover the basics.
The biggest confusion we see is to do with the naming of EpiPen. So let’s clear things up. Epipen is just a brand name - a type of adrenaline auto-injector. They’ve done incredibly well to become the household name most people refer to as they’re the most prescribed pen on UK market - think of it just like how Hoover dominated the vacuum cleaner space.
“I have lived with a severe nut allergy since I was about 5 year old, and have been prescribed all three brands of adrenaline pen over the course of the last 20 years. Often times I have found that both doctors and pharmacies don’t know the difference between the brands, or that there even are brands - they just prescribe ‘Epipen’ and you get what you get. This creates problems as then people can be prescribed new brands of pens they haven’t used before, but they aren’t told that it’s any different.”
- Zak Marks, Co-Founder of Kitt Medical
What is an adrenaline auto-injector?
An adrenaline auto-injector (often referred to as an AAI or adrenaline pen) is a medical device used to deliver a dose of adrenaline (epinephrine) in case of a severe allergic reaction, such as anaphylaxis. Adrenaline pens are designed to be easy to use and portable, and they are typically prescribed for individuals who have a known history of severe allergic reactions or anaphylaxis.
Adrenaline pens come in various brands and formulations, but they all work by injecting a measured dose of epinephrine into the muscle of the thigh. Epinephrine works quickly to counteract the effects of the allergen and can help to relieve symptoms such as breathing difficulties, swelling, and low blood pressure.
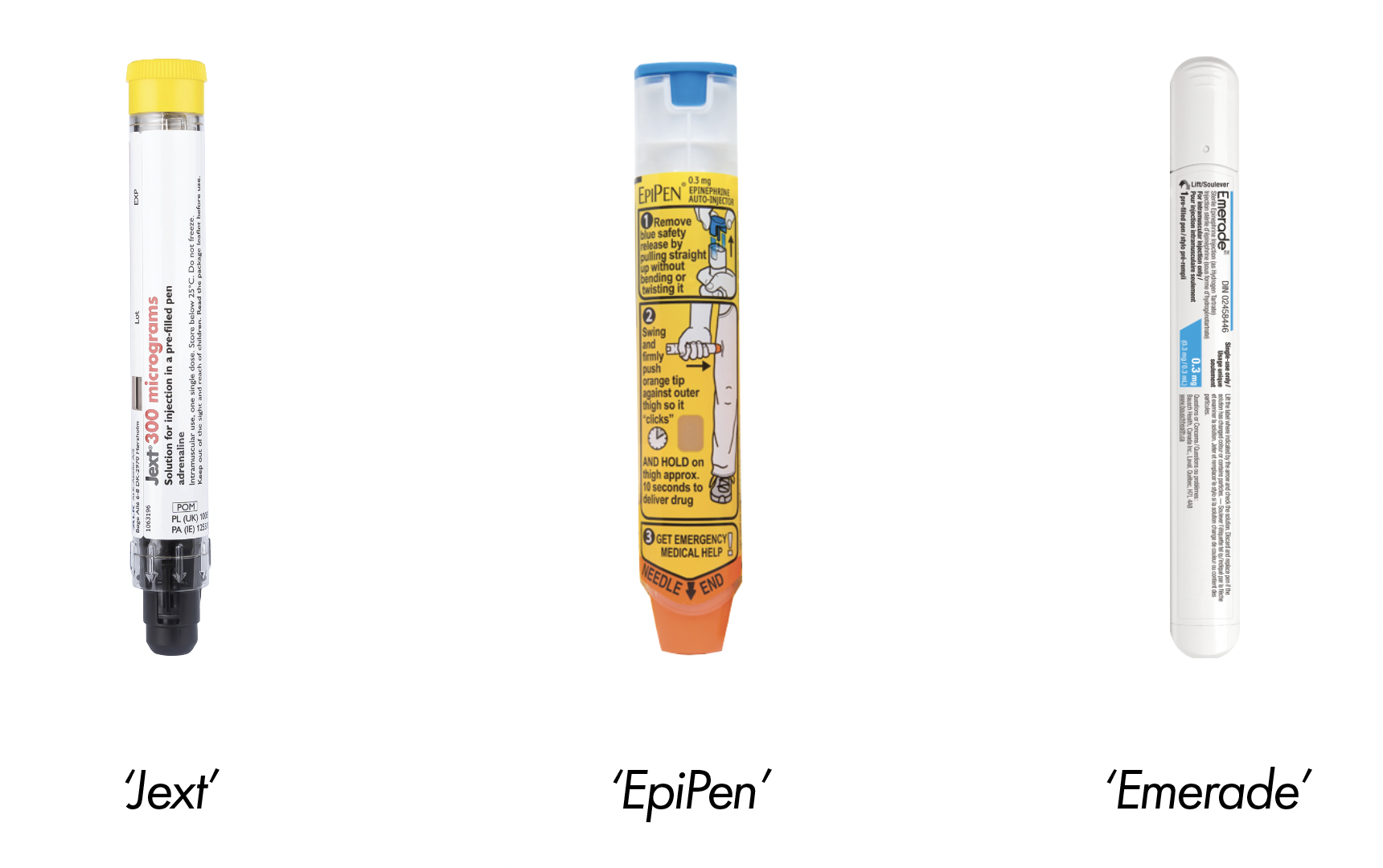
In the UK there are three different brands of adrenaline pens which are equally prescribed to individuals with severe allergies (anaphylaxis): EpiPen, Jext, and Emerade.
EpiPens and Jext pens come in 150mcg and 300mcg doses, whilst Emerade comes in 300mcg and 500mcg doses.
It’s worth noting that there is evidence to suggest that a single 300mcg EpiPen or Jext pen is a suitable replacement for a single Emerade 500mcg pen. This is based on recently available results from a study which compared blood levels of adrenaline following injection of Emerade 500 microgram pens with those following EpiPen 300 microgram or Jext 300 microgram pens. For more information on this, watch or read our interview with Professor Adam Fox on Allergy, Anaphylaxis & Adrenaline.
So - what’s with this recall?
On the 9th of May 2023, the UK government’s Medicines and Healthcare Product Regulatory Agency (MHRA) published a press release issuing a patient-level recall of Emerade pens.
In the press release, they stated that “some 300 microgram and 500 microgram Emerade auto-injector pens may rarely fail to activate if they are dropped, meaning a dose of adrenaline would not be delivered.”
They also mentioned that “premature activation has also been detected in some of the 300 microgram and 500 microgram pens after they have been dropped, meaning that a dose of adrenaline is delivered too early. The activation failure and premature activation was detected during a design assessment conducted by the manufacturer and therefore means there is a potential for some 300 microgram and 500 microgram Emerade pens to fail during use after having been dropped.”
For reference, this will be the fourth time Emerade has had issues and has been required to recall some or all of its stock:
March 4th 2020: All 150mcg Emerade auto-injectors were recalled
April 7th 2020: All 300mcg Emerade auto-injectors were recalled
May 18th 2020: All 500mcg Emerade auto-injectors were recalled
And now on 9th May, 2023: All 300mcg & 500mcg Emerade are recalled
This recall has been issued directly by Pharmaswiss Česka republika s.r.o. and distributor Bausch & Lomb UK Limited. They will now have to prove to regulators that their product is up to standard and has overcome the aforementioned issues before returning to market.
Dr Alison Cave, MHRA Chief Safety Officer, said about the recall:
“Patient safety is our top priority.
We are taking prompt action to protect patients, following detection of damage to internal components of the Emerade pens if they are dropped, which may mean they activate too early or fail to activate and deliver adrenaline.
The Department for Health and Social Care has confirmed that there are appropriate supplies of EpiPen or Jext adrenaline pens available for patients across the UK, however, patients will need to request a new prescription.”
So who does this concern?
Patients, or carers of patients, who carry Emerade 300 or 500 microgram adrenaline auto-injector pens should contact their GP and obtain a prescription for, and be supplied with an alternative brand. They should then return their Emerade 300 or 500 microgram pens to their local pharmacy.
This recall does not directly affect any Kitt Medical customers as we don’t directly supply Emerade through our Anaphylaxis Kitt service for schools.
Who are Kitt Medical?
Our multi-award-winning Anaphylaxis Kitt service is just like a defibrillator, but for allergies - providing schools with a regulated supply of emergency adrenaline pens, unlimited access to our CPD-accredited online training course, and more – all in one convenient annual subscription.
To find out more about our Anaphylaxis Kitt service for schools, click here.